

To be able to calculate the moles we need to look at a periodic table and see that 1 mole of C weighs 12.0 g and H weighs 1.0 g. To solve this problem, it is necessary to determine how much oxygen should be added if all of the reactants were used up (this is the way to produce the maximum amount of CO 2).įirst, we calculate the number of moles of C 2H 2 in 6.0 g of C 2H 2. If she uses the equation below, how much oxygen should she add to the reaction?ĢC 2H 2(g) + 5O 2(g) -> 4CO 2(g) + 2 H 2O(l) Often, it is necessary to identify the limiting reagent in a problem.Įxample: A chemist only has 6.0 grams of C 2H 2 and an unlimited supply of oxygen and he desires to produce as much CO 2 as possible. Sometimes when reactions occur between two or more substances, one reactant runs out before the other.
How many moles of Ca are in 4.50 grams of Ca?Ĭitation: Department of Chemistry at UNC Chapel Hill at Molar Mass of Ca = 40.08 (From the Periodic Table) So, 40 grams of calcium makes one mole, 80 grams makes two moles, etc. For example, calcium has an atomic mass of 40 atomic mass units. Given the atomic or molecular mass of a substance, that mass in grams makes a mole of the substance.
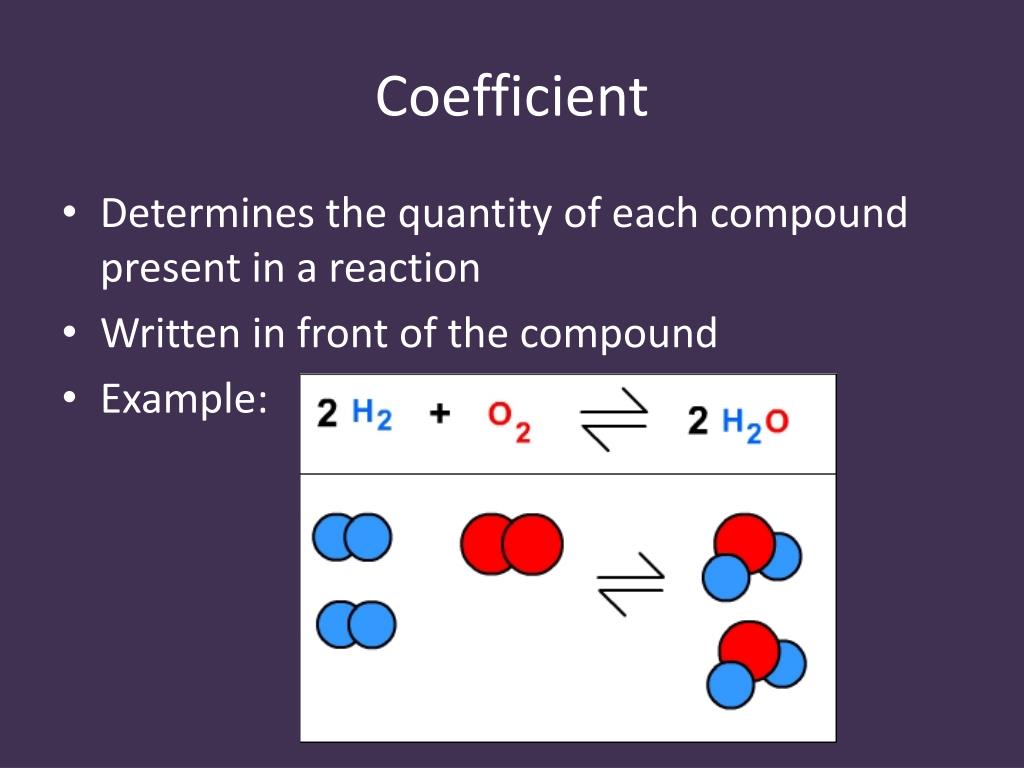
This conversion can be easily done when the atomic and/or molecular mass of the substance(s) are known. Thus, you have the same number of moles of AgNO3, NaCl, AgCl, NaNO3.Ĭonverting between moles and grams of a substance is often important. In the equation above there are no numbers in front of the terms, so each coefficient is assumed to be one (1). Similarly, if you have a mole of carrots, you have 6.022 x 1023 carrots. If you have a dozen carrots, you have twelve of them.

A mole is similar to a term like a dozen. Given enough information, one can use stoichiometry to calculate masses, moles, and percents within a chemical equation.Ī mole simply represents Avogadro's number (6.022 x 1023) of molecules. % error = | 120.104 g/mol - 120.056 g/mol | / 120.Stoichiometry is simply the math behind chemistry. % error = | theoretical - experimental | / theoretical * 100% Check your result by calculating the molar mass of the molecular formula and comparing it to the experimentally determined mass.Įxperimentally determined mass = 120.056 g/mol Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula.Ħ. If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass.ĥ. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. Divide the experimentally determined molecular mass by the mass of the empirical formula. Determine the molecular mass experimentally. In the example above, it was determined that the unknown molecule had an empirical formula of CH 2O.ġ. (1/0.0332)(0.0333mol C : 0.0665mol H : 0.0332 mol O) => 1mol C: 2 mol H: 1 mol Oįrom this ratio, the empirical formula is calculated to be CH 2O. (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol OĬonstruct a mole ratio for C, H, and O in the unknown and divide by the smallest number. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O 2 reactant.Ġ.333mol CO 2(44.0098g CO 2/ 1mol CO 2) = 1.466g CO 2ġ.466g CO 2 + 0.599g H 2O - 1.000g unknown organic = 1.065g O 2ġ.065g O 2( 1mol O 2/ 31.9988g O 2)( 2mol O/ 1mol O 2) = 0.0666mol O Using the Law of Conservation, we know that the mass before a reaction must equal the mass after a reaction. This will give you the number of moles from both the unknown organic molecule and the O 2 so you must subtract the moles of oxygen transferred from the O 2.Ġ.0333mol CO 2 ( 2mol O/ 1mol CO 2) = 0.0666 mol OĠ.599g H 2O ( 1mol H 2O/18.01528 g H 2O)( 1mol O/ 1mol H 2O) = 0.0332 mol O Since all the moles of C and H in CO 2 and H 2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample.Ġ.0333mol CO 2 ( 1mol C/ 1mol CO 2) = 0.0333mol C in unknownĠ.599g H 2O ( 1mol H 2O/ 18.01528g H 2O)( 2mol H/ 1mol H 2O) = 0.0665 mol H in unknownĬalculate the final moles of oxygen by taking the sum of the moles of oxygen in CO 2 and H 2O.


 0 kommentar(er)
0 kommentar(er)
